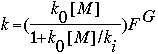
CNumbers allows the mandatory naming of the non-methane organic species included in the VOC mixture, and tells OZIP the number of carbon atoms in each species. The species named in CNum must match the VOC species listed in the BOUNdary/REACtivities option. Also, this option must precede the REACtion listing on input.
The REACtion listing format follows these rules:
Immediately after the CNUM option, the text string "REACTIONS =" designates the beginning of free-format reaction listing input. This is followed by a listing of each consecutive reaction as described next. Species names have up to 4 significant characters, any extra are truncated. Legal characters to use in naming species are "A-Z", "a-z", "0-9", and ":". Tabs are not allowed (the space bar must be used to insert blank spaces).
Each reaction begins with the reactants (if there are any). Zero to 3 reactants may be included, separated by "+". No stoichiometry is allowed for reactants. After the reactants, a "=" indicates that a list of products will follow. M, N2 and O2 may be used as reactants. The concentrations of these species have been coded into OZIP and do not need to be specified by the user (DF: O 2 = 209,460 ppm, N 2 = 780,84027 ppm, and M = 1,000,000 ppm). The rate constants given will be appropriately multiplied by the concentrations of these species to produce rate expressions.
Even if there are no products or no reactants, a "=" is mandatory for each reaction. Zero to twelve products and product coefficients are allowed. Products and their stoichiometric coefficients are joined by a "*". Products, or coefficient*product pairs, are linked by a "+". In the event of a negative coefficient, the "+" is still required prior to the "-" of the coefficient ("+" is a symbol linking entities while "-" is part of the coefficient).
After listing the reactions, a rate expression, initiated by either "#" or "%", must be included. There are many types of rate expressions, which are described below. Further, the rate expression may be modified by a number of options, including dependence on the solar zenith angle (photolysis reactions) and dependence or equilibrium with other rates or reactions. These modifiers begin with the symbols "*" and "/", followed by symbolic language as described below.
Each reaction is terminated by ";".
Rate Expressions are of two types, standard and special. Most standard expressions, with the exception of falloff expressions, can be used when rate constants are expressed in either CM or PPM units. The input of standard rate expression parameters begins with "#". Following this, the "@", "^", and "&" are used to allow input of various parameters as follows:
| Standard Expression | Input Form |
| k = A | #A |
| k = A x (T/300)B | #A^B |
| k = A x e(-C/T) (where C=Ea /R) |
#A@C |
| k = A x (T/300)B x e(-C/T) | #A^B@C |
The falloff expression listed below requires the values of A, B, and C to be input in CM units even if all other reactions are listed in PPM units.
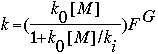 |
#Ao^Bo@Co&Ai^Bi@Ci&F&n |
where: ko and ki are of the form A x (T/300)B x e(-C/T) , or any other standard format.
and,
G = {1+[log(ko [M]/ki )/n]2 } -1
[M] is known and automatically included in the falloff rate constant calculations. Ea is the activation energy in kcal/mole. The value included on input is C = Ea /R, where R is 0.0019872 in these units. Be careful of the sign of C.
The falloff parameters ko and ki are input in one of the standard formats given above. ko always precedes ki on input. F and n need not be included. If not specified, the defaults of 0.6 for F and 1.0 for n are used. Units of these falloff reactions are always CM.
Special rate expressions are fixed algebraic expressions of a non-standard type. These expressions begin with the character "%"
.
| k = A x (1.0 + 0.6*Pressure) | %1 #A |
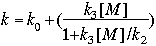
| #Ao@Co&A2@C2&A3@C3 |
where, ko , k2 , and k3 are of the form A x e(-C/T), as in the standard expression above.
Special rate 1 is used for the OH + CO reaction where pressure is in atmospheres and the value for A is either in CM or PPM depending on the main units in the MECH option.
Special rate 2 is a Lindemann-Hinshelwood expression, primarily used for the OH + HNO3 reaction. These parameters must alwaysbe in CM units.
Modifiers of rate expressions provide (1) dependence on another rate, (2) reverse equilibrium with a second-order forward reaction, or (3) dependence on a value that varies with zenith angle of the sun.
Linear dependence on another rate is of the form:
| k = A x Kn | #A *Kn |
where A is a linear factor and Kn is the nth reaction listed in the MECH option. This expression can be used in either rate format (CM or PPM).
A reverse equilibrium rate (only for first-order decomposition) can be calculated directly from the equilibrium constant (K eq ) by including the equilibrium constant data and the reaction number of the forward equilibrium reaction.
| kr = kf / A x e(-C/T) | #A@C *En |
where A@C is the equilibrium constant and n is the reaction number of the forward reaction. This expression can be used in either rate format (CM or PPM), with any type of forward rate expression, but the equilibrium expression must be in the "A@C" format and the reverse reaction must be a first-order process.
Photolysis reaction indicators follow the kinetic expressions for each reaction. The format is either "/Ln" or "/Rn", where L refers to a specific table (Ln) of absolute reaction rate factors vs. solar zenith angle in the ZENI option and R refers to table Rn of relative reaction rate factors in ZENI. The relative factors will be multiplied by the absolute factors listed for L1 in the ZENI option, after which, all relevant factors will be multiplied by the calculated rate of reaction. For this reason, in both the REACTION listing and the ZENI option, the listing of L1 should precede the listing of any relative (R) reaction rates. Note that first-order reaction rates are in min -1 for PPM units and sec -1 for CM units.
The rate modifiers operate under the following restrictions: (1) "*K" and "*E" cannot be used in the same reaction, (2) any "*" modification must precede "/" if in the same reaction, and (3) photolytic ("/L" or "/R") reactions are allowed with falloff rate expressions.
 The Shodor
Education Foundation, Inc.
The Shodor
Education Foundation, Inc.